Non-isothermal kinetics for crystallization, curing and reactions has been being a research topic for more than half a century.
This is because non-isothermal kinetics involves an equation termed Arrhenius Equation, that states the reaction rate constant
is an exponential function of the minus activation energy divided by the product of the gas constant and temperature,
as shown below:
$$k = A \; exp \left ( {-E_{a}} \over {R \; T} \right ) \tag{1}$$
where, k — the rate constant; A — pre-exponential factor also known as frequency factor; Ea
— activation energy; R — gas constant [8.314 J/(mol K)] and T — temperature.
Equation (1) appears simple but there is no analytical solution that can be devised so far in spite of constant challenging
endeavours. On the other hand, the kinetics parameters are an absolute necessity in order to make the degree of reaction,
curing and crystallization calculable/estimable.
n-th Order Kinetics Models
Most existing methods were developed by assuming a n-th order reaction:
$$ \frac{d \alpha}{dt} = k ( 1 - \alpha) ^ n \tag{2} $$
where, α — degree of reaction; t — time; n — order of reaction.
The Kissinger Plot:
Kissinger took the deritive of Eq.(1) and Eq.(2) and assumed that the reaction rate (namely dα/dt) reaches maxium at the
temperature (Tp) where DSC curve displays the peak. i.e.
$$ \begin{aligned} \frac{d \left ( \frac{d \alpha}{dt} \right )}{dt} &= A \; exp \left (\frac{-E_a}{R \, T} \right) \frac{E_a} {R \,T^2} \left ( 1-\alpha \right ) ^n \frac{dT}{dt} \; - \; n(1-\alpha)^{n-1} \: A \; exp \left (\frac{-E_a}{R \, T} \right) \frac{d \alpha}{dt} \\ &= 0 \end{aligned} \tag{3} $$
By further assuming n(1-α)(n-1) is a number close to unit, and dT/dt =β
(heating rate) is a constant, Kissinger reached:
$$ ln \left ( \frac{\beta}{T_p ^2} \right ) = ln \left ( \frac{A \, R}{E_a} \right ) \; - \; \frac{E_a}{R \, T_p} \tag{4} $$
The Kissinger plot thus says that for a given DSC curve with the heating rate, β, one observes the maxium reaction rate
at the peak temperature, Tp; for a set of DSC curves with different heating rates, one can plot the quantity of
ln(β/Tp2) against 1/Tp to obtain the Kissinger plot. From the slope of the
Kissinger plot, one in turn obtains the activation energy, Ea; futher from the intercept one obtains the
pre-exponential factor, A, as well.
Steps for determination of activation energy, Ea, from the Kissinger plot
1) Run DSC experiments with several different heating rates.
2) Find the value of temperature at which the DSC curve reaches its peak, Tp;
3) Convert the peak temperature to from (°C) to (K) by adding 273.15;
3) Calculate ln(β/Tp2) (β can be in unit °C/min, or any other units
as long as the same unit is used);
5) Plot ln(β/Tp2) against 1000/ Tp; use linear regression
method to determine the slope of the plot;
6) Calculate by multiplying the absolute value of the slope by R and by 1000 as follows:
$$ E_a = 1000 \times 6.9016 \times R = 1000 \times 6.9016 \times 8.31 = 57380 \;
(J/mol)
$$
The Ozawa Plot:
Incoorporating with various results through thermal analysis research, late Prof. Ozawa proposed following equation:
(ref: Ozawa T. "A New Method of Analyzing Thermogravimetric Data". Bull. Chem. Soc. Jpn. 1965;
38(11): 1881-1886;
doi:10.1246/bcsj.38.1881)
$$ ln \left ( \beta \right ) = const \; - \; 1.052 \: \frac{E_a}{R \, T_p} \tag{5} $$
where, β — heating rate; Ea — activation energy; R — gas constant;
Tp — peak temperature.
According to Eq. (5), we can run several DSC experiements with different heating rates; observe the peak and determine the
peak temperature for each DSC curves. Ploting ln(β) against 1/Tp, we obtain the Ozawa plot. The activation
energy can be determined from the slope of the Ozawa plot.
ASTM E698 Thermal Stability is based on the theory of the Ozawa plot. Typically, three or more experiments are required
with different heating rates between 1 and 10°C/minute.
An example how the Ozawa plot is obtained from the DSC curves for an epoxy resin curing is shown below:
Steps for determination of activation energy, Ea, from the Ozawa plot
1) Run DSC experiments with several different heating rates.
2) Find the value of temperature at which the DSC curve reaches its peak, Tp;
3) Convert the peak temperature to from (°C) to (K) by adding 273.15;
4) Calculate ln(β) (β can be in unit °C/min, or and other units as long as the same unit is used);
5) Plot ln(β) against 1000/ Tp; use linear regression method to determine the slope of the plot;
6) Calculate Ea as follows:
$$ E_a = \frac{1000 \times 7.8023 \times R}{1.052} = \frac{1000 \times 7.8023 \times 8.31}{1.052} = 61662 \; (J/mol)
$$
The Borchardt and Daniels (B/D) Method:
From Eq. (1) and Eq. (2), Borchardt and Daniels simply took logarithms leading to:
$$ ln \left ( \frac{d \alpha}{dt} \right ) = ln \left ( A \right ) \; - \; \frac{E_a}{R \, T}
+ n \: ln(1-\alpha) \tag {6a}
$$
or
$$ ln \left [ k(T) \right ] = ln \left ( A \right ) \; - \; \frac{E_a}{R \, T} \tag{6b}
$$
One then determines a and da/dt with a tentative n to obtain ln[k(T)] from a DSC curve for 20 curve segments evenly spaced
by temperature starting at 10 percent of the peak height and ending at 50 percent of the peak area. A plot of ln[k(T)]
versus 1/T should be a straight line by adjusting the tentative value for n. The activation energy, Ea,
and pre-exponential factor, A, are obtained from the slope and intercept of this plot respectively.
Model Free Kinetics (MFK)
Minding of limitations of the nth order reaction models, researchers have been making attempts to establish model free kinetics
analysis methodology, in which a generic form function f(α) is used to replace the function of the nth order
(1-α)n. However, new assumption(s) is/are imposed, adding a new dimension of uncertainty to
the analysis results.
$$ \frac{d \alpha}{dt} = k(T) \, f(\alpha) = A \; exp \left (\frac{-E_a}{R \, T} \right) \, f(\alpha) \tag{7} $$
Friedman's method:
Taking logrithms for Eq.(7) leads to:
$$ ln \left ( \frac{d \alpha}{dt} \right ) = ln[f(\alpha)] + ln(A) \; - \; \frac{E_a}{R \, T} \tag{8} $$
Under the isoconversion assumption, the function f(α) reaches a given value thus a constant. Therefore the plot of
ln(dα/dt) against 1/T results in a straight line with the slope being -Ea/R.
Ozawa-Flynn-Wall method:
Ozawa, Flynn and Wall tried to rewrite Eq. (7) in an integral form, followed by a replacement of
the integrant by an approximation function. This treatment has led to establishment of the following equation.
$$ ln(\beta) = ln \left ( \frac{A E_a}{R} \right ) - G(\alpha) - 5.3305 - 1.052 \frac{E_a} {R \, T} \tag{9} $$
Under the isoconversion assumption, i.e. the function G(α) reaches a given value thus a constant. Therefore the plot
of ln(β) against 1/T results in a straight line with the slope being -1.052 Ea/(RT).
This may constitute of a theoretical proof why and how the Ozawa plot (Equation (5) as shown above) is correct.
Vyazovkin and Wight's Method:
This method obtains the activation energy and the pre-exponential factor by computing the minimum of the sum of product of
an Arrhenius integral and heating rate over a number of different heating rates.
DCS' Novel Methodology
What do we need? We need to know the activation energy, Ea, and pre-exponential factor, A, and all the relevant parameters
as well as the reaction model so that we can calculate the degree of reaction for any given thermal history - the usefulness of
a methodology lies in its predictive power !
Beyond the n-th order model: Though the nth order model does describe a mechanism for a number of reactions, it is
not good enough for many other cases. For instance, we often prefer to use the autocatalytic model for expoxy curing;
the Avrami model for crystallization, and so on and so forth.
DSC Curve Solutions (DCS) deals with non-isothermal kinetics in a different approach, a trial and error approach -
it gives tentative figures of all the kinetic parameters first to enable computation of the whole DSC curves, compares
these simulated DSC curves with the experimental curves, then gives new tentative figures until you are satisfied with
curve fitting of the experimental DSC curves with DCS simulated DSC curves. In this manner, DCS determines all the
kinetic parameters from any single DSC run. When you have several DSC runs with different heating rates, you will find that
the kinetic parameters determined from any particular single run fit for other runs as well -astonishingly amazing !
superior predictive power !
Why and how can DCS achieve this?
• DSC Curve Solutions takes advantage of all information contained in a DSC curve (i.e. all the points on curve)
to capture the kinetic and model parameters; in comparison with the conventional methods that use only a few featured points
such as peak temperature and isoconversion points to extract kinetic parameters.
• Secondly, DSC Curve Solutions integrates non-isothermal kinetics analysis and DSC curve simulation into one; this means,
DCS deals with kinetics as one thermal event only, a DSC curve is the combined results of all the thermal events
involving in a DSC experiment.
An example:
Adam wants to know the kinetic parameters for an epoxy resin. He heats the epoxy to 290°C at 5°C/min,
and obtains a curing exotherm DSC curve shown in black. Using the autocatalytic model, Adam readily determines the
kinetic parameters of the epoxy resin by fitting this SINGLE run DSC curve (black) with the DCS curve (red) to satisfaction.
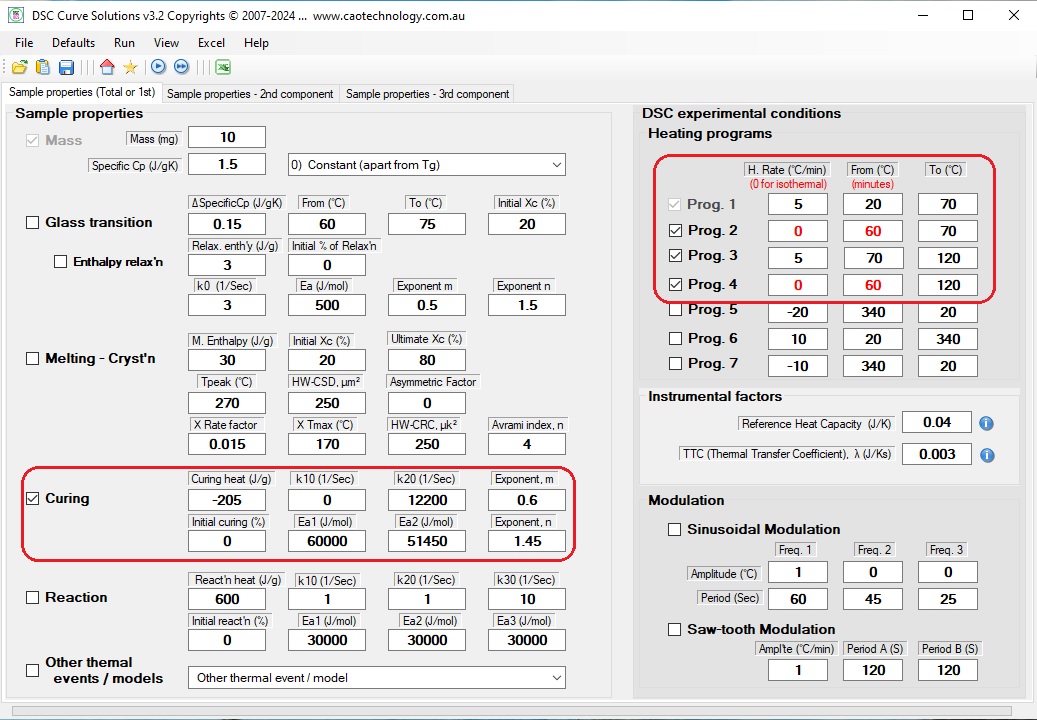
Curing enthalpy: ΔH = -205 J/g;
Frequency factor: k10 = 0
(Activation energy, Ea1 can be any in this case);
Frequency factor: k20 = 12200 s-1;
Activation energy: Ea2 = 51450 J/mol;
Exponent: m = 0.60;
Exponent: n = 1.45
Autocatalytic model:
$$ \frac{d \alpha}{dt} = \left ( k_1 + k_2 \, \alpha ^m \right ) (1-\alpha) ^n \tag{10}
$$
where:
$$ k_1 = k_{10} \; exp \left ( \frac{-E_{a1}}{R \, T} \right ) \tag{11}
$$
$$ k_2 = k_{20} \; exp \left ( \frac{-E_{a2}}{R \, T} \right ) \tag{12}
$$
Now, using DSC Curve Solutions, we have leant that the resin's curing behaviour is described as:
$$ \frac{d \alpha}{dt} = 12200 \;\; exp \left ( \frac{-51450}{R \, T} \right ) \; \alpha ^{0.6} \; (1-\alpha)^{1.45} \tag{13} $$
Equation (13) is exactly what we want, no more and no less.
To have a free trial of DCS, simply click Free trial
Q1: The resin was unintentionally left at 70°C for an hour, how much degree of curing it has experienced?
Q2: The resin is subsequently heated to 120°C at 5°C/min, what is degree of curing?
Q3: The resin is cured at 120°C for an hour, is it fully cured?
To answer these questions, fill the parameters to the DCS, and set heating programs as shown below:
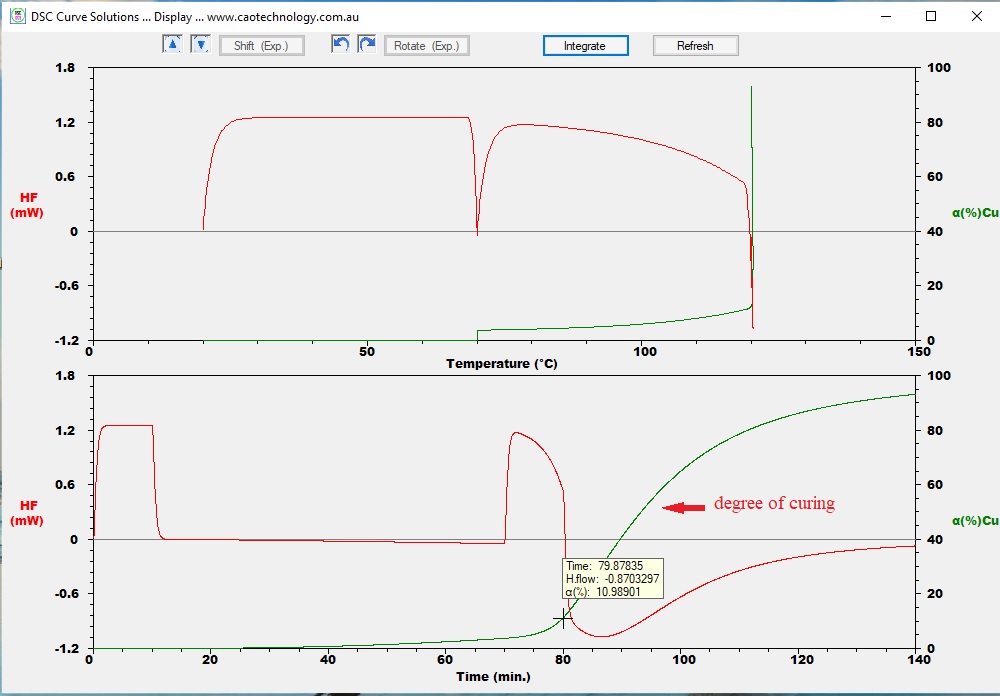
Run a simulation, one obtains DSC curves of heat flow vs. tempelature and of heat flow vs. time. From the curve of curing degree vs time,
one obtains that unintentional stay at 70°C for an hour resulted in 3.27% curing; the degree of curing reached 10.9% when being heated to 120°C,
and finally reached 92.9% of curing.
Alternatively, generic commercially available mathematics software, e.g. MatLab, Mathmatica can be used to solve the differential
equation to calculate answers as long as all the parameters for the autocatalytic model are known. However, VisualLab, a universal syntax free
new generation powerful mathematics software also developed by CaoTechnology, can be even more convenient and useful. Simply type
in the equation and parameters in, click Button Solve, one learns that the resin has cured 3.27% by exposing to 70°C for an hour.
The graph below is a screenshot showing how easily VisualLab solves this question. Note that a very small number, 0.0000001 in this
case (can be any) is required for the initial curing. This is a feature of the autocatalytic model - cannot be 0.
One obtains degree of curing 3.27% for Q1. Solving the differential equation again by altering the heating condition and initial condition
as shown in the following screenshot, one obtains: 10.88%.
Solving the differential equation again by setting temperature as a constant of 120°C,
and inputing corresponding initial condition into the box. The result displayed in the following
screenshot shows that the resin is 92.9% cured, somehow undercured, indicating that a longer
curing time would be better if >95% curing is required. The curing graph, a plot of degree
of curing, α, vs. time (seconds), is also shown below.
To have a free trial of DCS, simply click Free trial